WARNING! Peppermints were harmed in the making of this blog post. Proceed with caution!

It’s that time of year when we are given many peppermint flavored treats! I am going to tell you a little secret. I didn’t liked peppermint flavored anything until the past year or so! Starting about a year ago, I suddenly didn’t mind a cup of peppermint tea. Now I love all the peppermint goodies I can get my hands on. In fact, my newfound love of all-things peppermint has inspired a few things lately, like these delicious vegan peppermint brownies I recently made.

While peppermint has a huge association with the winter holidays, it is used year round and has a plethora of applications, such as:
- potential treatment for irritable bowel syndrome
- use as a mosquito repellant
- muscle and mental relaxation effects
- limiting the spread of some harmful bacteria
What are some of the key chemicals that give peppermint it’s distinct flavor and scent? A little hint actually comes from the history of the peppermint plant, which is actually a hybrid between the watermint and spearmint plants.
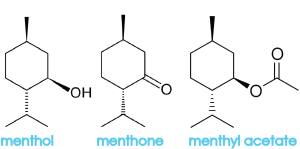
Menthone and menthyl acetate belong to a class of molecules called terpenes, which are often strong smelling and key components in essential oils. Menthol, an alcohol, is actually derived from the terpene, limonene. Oils extracted from mint plants contain menthol and menthone, which is what makes these plants taste minty. What gives them their distinct differences are the relative amounts of menthol and menthone, as well as the amounts of other trace chemicals that are present. Here are some additional chemicals that can be found in peppermint oil:
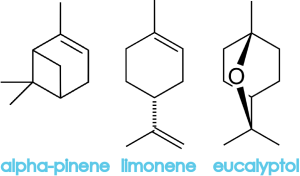
Food for thought: Because of the high concentration of menthol in peppermint oil, menthol has a similar effect to capsaicin. Capsaicin creates a burning sensation when it touches the skin and menthol creates a cooling sensation. Check out this great blog post by the Boundless Thicket if you want to learn why menthol makes you cool.
Whew. I need a mint!

HAPPY HOLIDAYS, From Team CAYF!
All images are ©ChemicalsAreYourFriends.com
